NYSCF Scientists Create the ‘Stars’ of the Brain and Capture Their Rogue Behavior in Disease
NewsRead the press release here.
You may not have heard of astrocytes before, but your body is full of them: they make up more than half the cells in your central nervous system. And while they are typically helpful for normal brain function, a new study in Neuron led by NYSCF Research Institute Senior Research Investigator Valentina Fossati, PhD, suggests that in neurodegenerative diseases like Alzheimer’s, Parkinson’s, or multiple sclerosis, these usually friendly cells may turn into foes.
Dr. Fossati’s team established a method for creating and identifying astrocytes from stem cells, leading to the first demonstration in human cells that inflammation can lead to neurodegeneration. This powerful model for studying how astrocytes can turn toxic to neurons opens a new avenue for strategies to treat these devastating diseases.
Making the ‘Stars’ of the Brain
Dr. Fossati describes astrocytes — named for their ‘star-like’ structure — as parents to the neurons they support.
“If a neuron is like a baby, then an astrocyte is like its mother,” she explains. “Astrocytes feed the neurons, clean the neurons, and keep the neurons healthy.”
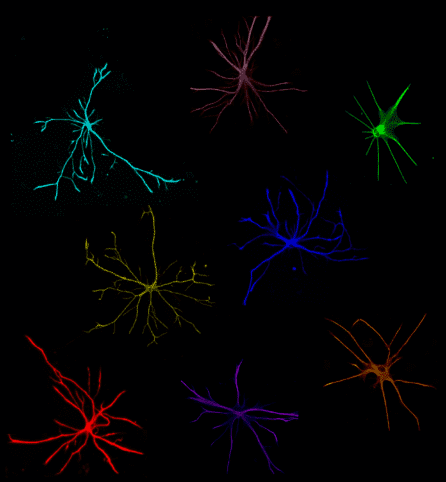
Mounting evidence has also suggested that astrocytes play a role in neurodegenerative diseases, but most of this work has been done in mice, and mouse astrocytes are not the same as human astrocytes. To truly understand how human astrocytes behave in human diseases, scientists need to study human cells. But they can’t just reach into a living brain and pull out the astrocytes. That’s where stem cells come in.
“With stem cells, we can make the actual human brain cells impacted by disease and see how they behave under different conditions,” says Dr. Fossati. “In this study, we devised an optimized method for creating astrocytes from stem cells, giving us a window into their role in disease and opportunities to correct their dysfunction.”
The key to Dr. Fossati’s new method for generating stem-cell-derived astrocytes is the discovery that a certain protein called CD49f is expressed in these cells. Using CD49f as a ‘tag,’ scientists can look at a pool of cells, determine which ones are astrocytes, and then pull them out and study them up close.
Dr. Fossati’s team was pleased to see that their stem-cell-derived astrocytes isolated with CD49f acted like typical astrocytes: encouraging neurons to form connections, cleaning up their environment, etc. Equipped with a new model for studying astrocyte behavior, the team then turned their attention to how astrocytes act in disease-like conditions.
When Astrocytes Go Rogue
In 2017, Dr. Fossati read a study of astrocytes that she found fascinating. The study, conducted by Stanford University scientists, found that in mice, astrocytes exposed to disease-like environments can turn toxic — attacking the neurons they typically support. She was inspired to contact Dr. Shane Liddelow, the lead author on the study.
“I thought, ‘Oh my god, this is cool. How can I translate this to a human system?’” recalls Dr. Fossati. “I reached out to Shane, who at the time was starting his own lab at NYU, and asked if he wanted to collaborate.”
“With the 2017 study, we really had no idea if the astrocyte changes we saw in rodents would be the same in human cells,” adds Dr. Liddelow, a collaborator on Dr. Fossati’s new study and Assistant Professor of Neuroscience and Physiology and of Ophthalmology at the NYU Grossman School of Medicine. “So, when Valentina reached out to me, she really hit the nail on the head as to this question we had been wanting to answer for years.”
To test whether the ‘rogue astrocyte’ phenomenon could happen in humans, Dr. Fossati took her healthy stem-cell-derived astrocytes and exposed them to an inflammatory environment — the kind that often exists in neurodegenerative diseases. This inflammatory exposure caused the astrocytes to change their shape (losing their characteristic ‘long arms’ and taking on a more constricted star-like shape) as well as to become worse at all of the jobs they typically do to support neurons. Dr. Fossati then collected the astrocytes’ byproducts and exposed these secretions to healthy neurons.
“Just like in mice, the neurons in the dish began to die,” says Dr. Fossati. “It was so exciting to see that what was happening in mice was also happening in human cells.”
Dr. Fossati is especially excited about what this result, and a new system for creating astrocytes, could mean for the future of disease treatment.
“This is a completely new mechanism that current treatments for neurodegenerative diseases haven’t targeted before,” she remarks. “I’m very optimistic that this, along with a new system for studying human astrocyte function, will open the door for effective drug discovery and bring new therapies to patients.”
Journal Article:
CD49f Is a Novel Marker of Functional and Reactive Human iPSC-Derived Astrocytes
Lilianne Barbar, Tanya Jain, Matthew Zimmer, Ilya Kruglikov, Jessica Sadick, Minghui Wang, Kriti Kalpana, Indigo V.L. Rose, Suzanne R. Burstein, Tomasz Rusielewicz, Madhura Nijsure, Kevin A Guttenplan, Angelique di Domenico, Gist Croft, Bin Zhang, Hiroko Nobuta, Jean M. Hébert, Shane A. Liddelow, Valentina Fossati. Neuron. 2020. DOI: https://doi.org/10.1016/j.neuron.2020.05.014