Women’s Reproductive Cancers Are Among The Most Deadly And Most Neglected. How Do We Fix This?
The American Cancer Society estimates that nearly 20,000 women will be diagnosed with ovarian cancer in 2022, and 12,000...
Our Approach News FAQs Meet the PI Scientific Advisory Board For Scientists Publications
Research on women’s reproductive cancers (such as ovarian cancer, endometrial cancer, uterine cancer, and cervical cancer) is massively underfunded given their deadly toll on society, and as a result they remain very challenging to treat. Over 100,000 women in the United States are diagnosed with reproductive cancers each year, and over 32,000 women die annually from these cancers. The NYSCF Women’s Reproductive Cancers Initiative aims to shift paradigms in the way these cancers are studied and treated, in collaboration with leading cancer experts across the globe.
Our work begins with ovarian cancer, one of the deadliest types among women as the 5th leading cause of cancer-related deaths. Five years after diagnosis, ovarian cancer survival rates across all subtypes and stages are just 47% – a number that has not changed in the past 25 years. For patients diagnosed with advanced ovarian cancer (more than 60%), 7 out of 10 will die within 5 years. This is partly due to the high frequency of patient relapses with tumors exhibiting resistance to therapies, making ovarian cancer extremely difficult to treat effectively. An ovarian cancer diagnosis is usually given at very late stages because of the vague symptoms and lack of effective ovarian cancer screening strategies.
A major limiting factor in understanding and treating ovarian cancer is the lack of experimental models that capture the particularities of each patient’s cancer. Moreover, cancer samples taken from patients have a finite lifetime which restricts what researchers can learn about each patient’s disease.
At NYSCF, we use samples of patient tumors collected during surgery, applying principles of stem cell technology to generate self-renewing tumor ‘avatars’ (called organoids) that recapitulate each patient’s individual tumor.
Our world-leading expertise in stem cell technology positions us to create these innovative, more effective, personalized models of cancer.
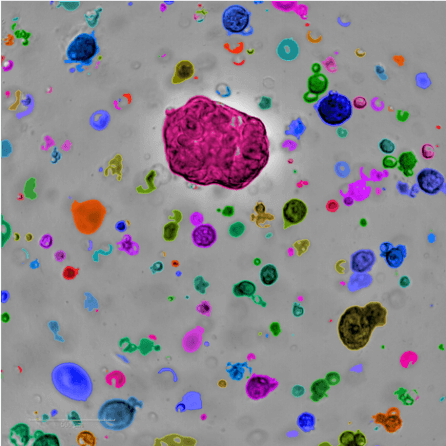
Tumor-derived organoids can continuously grow in the laboratory. This means we can study tumor cell behavior, test drugs, and identify tumor sensitivities to treatments, ultimately helping doctors choose the optimal therapy for each patient. At NYSCF, in collaboration with leading clinicians and researchers, we are building a large living biobank of ovarian cancer organoid models as a resource to advance the field and accelerate the development of more efficient treatments for patients.
In addition to the major unmet medical need for more advanced and innovative research solutions for these cancers, increased awareness of these issues is crucial for improving diagnosis, survival rates, and treatment effectiveness. NYSCF regularly holds scientific and patient-focused events that educate the public about these issues and present the latest advancements in women’s cancer research.
The American Cancer Society estimates that nearly 20,000 women will be diagnosed with ovarian cancer in 2022, and 12,000...
Ovarian cancer is a difficult disease to tackle, as is evident from the statistics that surround it: Five years...
How do we help our community to realize the full potential of stem cell science? To make progress in...
Ovarian cancer causes are not well established, but we do know that certain risk factors make someone more likely to develop the disease. For example, several genetic mutations (inherited or acquired over time) can contribute to its onset; we know the cancer starts in cells at the tail ends of the fallopian tubes or in the ovary itself; and cancer development may be related to ovulation.
Ovarian cancer risk factors include:
Genetic mutations in genes such as BRCA1, BRCA2, PTEN, PALB2, and RAD51
Aging (the risk of developing ovarian cancer increases with age, with most cases occurring after menopause)
Obesity
Taking hormone therapy after menopause
Having family history of ovarian cancer
Having a history of breast cancer
Smoking and alcohol use
Ovarian cancer stages range from 1-4, with higher numbers indicating more advanced stages. Ovarian cancer treatments often depend on the stage of the disease, and each stage is defined by the size of the tumor, its spread to nearby lymph nodes, and its spread to more distant sites.
Ovarian cancer signs and symptoms may include the following:
Pain in the abdomen or pelvis
Bloating
Fluid in abdomen
Indigestion
Nausea
Fatigue
Loss of appetite
Lump in abdomen
Weight loss
Frequent urination
Constipation or menstrual changes
The risk for ovarian cancer is influenced by genetics, and has been shown to increase as more family members are affected by the disease. Specifically, genetic mutations in genes such as BRCA1, BRCA2, PTEN, PALB2, and RAD51 can increase one’s risk of developing ovarian cancer.
Like most cancers, ovarian cancer tumors have the capacity to metastasize (move to other parts of the body). Common areas for ovarian cancer tumors to metastasize include the liver, the fluid around the lungs, the spleen, the intestines, the brain, skin, or lymph nodes outside of the abdomen.
While patients can go into remission from ovarian cancer following surgery and chemotherapy, there are no treatments that currently constitute a cure.
 Laura Andres-Martin, PhD
Laura Andres-Martin, PhD
Senior Research Investigator, Oncology
The NYSCF Research Institute
Laura Andres-Martin, PhD, is the founding Principal Investigator for the NYSCF Women’s Reproductive Cancers Initiative, which aims to shift paradigms in the way women’s cancers are studied and treated. Her team has built a growing biobank of ovarian cancer patient samples to fuel the discovery of precision therapies, and she hopes her work will help accelerate solutions for those suffering from this understudied disease.
Dr. Andres-Martin received her PhD from the University of Salamanca in Spain where she worked on deciphering the role of NTRK1 variants in cancer and rare disorders. She then worked as a postdoctoral fellow at Weill Cornell Medicine, focusing on neurodegenerative disorders and stem cell competition mechanisms in adult stem cells. In 2015, she was awarded a NYSCF – Druckenmiller Postdoctoral Fellowship and has been an integral member of the NYSCF community ever since.
Email: landresmartin@nyscf.org
The Initiative is guided by a Scientific Advisory Board of world-leading experts. Current members include:
[ninja_tables id=”12273″]
Special Advisor:
[ninja_tables id=”12379″]
For the most part, cancer is still treated with a one-size-fits-all approach (i.e. chemotherapies), but recent scientific breakthroughs are starting to enable tailored treatments that bring precision medicine approaches to the oncology space. Genomic-guided treatments have provided significant benefits to certain patients with varying ranges of success depending on the tumor type/alteration, but on average these approaches have only benefited less than 10% of patients. We need precision therapies driven by function, not just genetics, and organoids offer an ideal model for this. Tumor organoids are made from stem cells present in the tumor tissue and grown in 3D: they can recapitulate each patient’s tumor biology, including preliminary evidence of predicting responses to therapy.

Our work focuses on ovarian cancer, due to its extensive unmet need. Over 100,000 women in the United States are diagnosed with reproductive cancers each year, and over 32,000 women die annually from these cancers. We leverage tumor organoids to address the following goals:
1. To build a biobank of clinically and genetically diverse organoid models from ovarian cancer patients as community resource
2. To use organoids as models to predict responses to therapies and find tumor-specific therapeutic vulnerabilities
3. To use organoids as a platform to accelerate the clinical development of candidate emerging therapies, like immunotherapies and combinatorial strategies
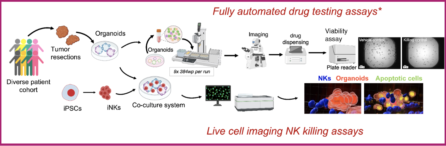
4. To leverage NYSCF’s world-leading automation to develop scalable assays for organoid therapeutic sensitivities. Automated methods significantly decrease technical variability, bringing reproducibility, robustness, and replicability to our assays while enabling scale and increased efficiency so that tumor-organoid based screenings can be readily adapted to large scale capabilities such as mid-/high-throughput screenings.
5. To ultimately enable the preclinical adoption of organoids-based therapy screening assays
1. Establishing highly efficient/robust methods for ovarian organoid derivation across subtypes
2. Building an ovarian cancer organoid biobank representing the most frequent ovarian cancer subtypes that is continuously expanding and available for community as resource
3. Establishing fully automated mid-throughput drug screening assays
4. Establishing a co-culture system with Natural Killer (NK) cells and 3D live-cell imaging killing assays to study and optimize responses to NK-cell based immunotherapies
5. Identification of a Taurine-mediated mechanism that suppress ovarian cancer cell growth and support the emergence of cells resistant to chemotherapy
1. Establishment of organoids cultures and biobanking from patients tumor resections
2. Histological and genomic characterization in matching primary tumor/organoid pairs
3. Mycoplasma testing in tumor organoid cultures
4. Organoid based in vitro assays such as proliferation and flow cytometry
5. Fully automated organoid-based drug testing
6. Evaluation of cytotoxic activity of NK-cell based immunotherapies
Ovarian Cancer: Towards Personalizing Ovarian Cancer Treatments Using Patient-Derived Organoids
Tatiana Volpari, Jacqueline Hebner, Raeka S. Aiyar, Laura A. Martin. Comprehensive Pharmacology. 2022. DOI: 10.1016/B978-0-12-820472-6.00080-3