90% of Drugs Fail Clinical Trials. How Can Stem Cells Improve Drug Discovery?
When it comes to clinical trials, current drugs are flunking out at alarming rates. Only one in ten will...When it comes to clinical trials, current drugs are flunking out at alarming rates. Only one in ten will graduate and make it to patients. The cost of developing them is astronomical (roughly $1 Billion each, in part because of how often they fail), and many likely were not the best candidates to begin with.
How do we reboot this process to get the right drugs to the right patients? Luckily, with the power of stem cell technology and patient models, scientists have new opportunities to identify drugs more effectively.
What’s Going so Wrong with Drug Discovery?
“The biggest issue we have in drug discovery is really choosing the right target for the disease,” noted Christine Brideau of Deerfield Management in a panel discussion on drug discovery held at this year’s NYSCF Conference. “Most drugs will fail in a Phase 2b trial because of lack of efficacy, and part of it is because maybe we didn’t choose the right target for the disease, or perhaps we didn’t have the right biomarker [measureable substance] to be able to determine whether the drug worked, or maybe we didn’t select the right patients.”
It’s hard to get all of these elements correct. Diseases have many different targetable features (some even invisible to the human eye), there are many ways to measure how a drug works, and every disease and drug affects a patient differently.

And that’s assuming we’re testing the most promising drugs to begin with. Many drugs advancing to clinical trials are discovered using mice, which don’t experience disease the same way humans do, or using cells from just a few patients who aren’t representative of the wider population.
We also have to move faster: we need methods for testing more compounds and quickly triaging out what doesn’t work before the drugs enter clinical trials.
“When you think about this normal V-shaped funnel that we always see in every drug discovery talk, we want to turn this into a T, where we broaden our search and fail faster so we can find what works,” added Tim Ahfeldt, PhD, of Recursion Pharmaceuticals.
Human Cells for Human Diseases
In addition to many diseases being studied in animals rather than humans, some are also studied in parts of the body where they may not originate, which limits our ability to find effective drugs. For example, PTSD has largely been studied in the blood, but the disease originates in the brain. With stem cells, we can access brain cells without surgery by creating them from stem cells.
“We recently published a study where we created a model of a PTSD-affected brain in the dish,” shared NYSCF Senior Vice President, Discovery & Platform Development Dan Paull, PhD. “We took stem cells from combat veterans exposed to trauma and turned them into different types of neurons.”
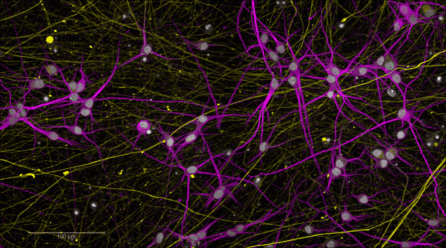
“We wanted to better understand why PTSD-affected cells hyper-respond to stress, so we introduced a stress hormone to these cells and found new features of PTSD-prone neurons. Additionally, having a robust stem cell model provides an ideal avenue to drug screening ‘in the dish,’ even across diverse patient populations.”
Robots to the Rescue
At NYSCF, we’ve developed technologies to scale up research, reduce human error, and find drugs faster. Our robotic platform, The NYSCF Global Stem Cell Array® can create high quality stem cells from hundreds of patients, and integrates analysis tools powered by artificial intelligence.
One of the advantages of this robotic system is its ability to produce stem cells that are highly standardized, helping ensure that the results of experiments are reliable.

“[When you make stem cells by hand], you can get all sorts of technical noise from people just doing things differently, having had coffee in the morning or not had coffee in the afternoon – little things like that. We turn to robotics to be able to help us overcome many of these aspects.”
Typically, creating just 10 stem cell lines by hand takes 4-6 months. The Array, however, can make much more, as well as run the analyses needed for large scale experiments.
“There’s thousands of cell lines individually growing everyday [on the Array],” remarked Dr. Paull. “All these cells get imaged, and then all that data has to get crunched and put into a database where we use it to run models.”
Many labs are now embracing automation, including that of Daniel Ramos, PhD, at the National Institute on Aging, whose work is aiming to understand the mechanisms behind dementia with stem cells.
“We’re setting up our automated cell culture system and trying to figure out the best way to run these experiments,” he said. “It seems like every time we run into a problem, NYSCF has also encountered that problem and solved it.”
When we have more stem cell lines available, we can also represent a broader population of patients, ensuring that findings don’t apply to just one group.
“[The Array] has allowed us to build things like a repository of cell lines,” noted Dr. Paull. “We have a lot of different diseases, ages, and ethnicities in our repository that are tied to different data sets, and this helps us deepen our understanding of disease.”
Widening the Net
Part of the idea behind accelerating drug discovery is, as Dr. Ahfelt mentioned, widening our search to look beyond the usual suspects of disease and leave room for surprise. Beth Cimini, PhD, of the Broad Institute, does this via a method called ‘Cell Painting.’ Typically when scientists study cells, they ‘tag’ certain structures or molecules that they suspect could be involved in a disease. Dr. Cimini, however, has turned to tagging major structures (called ‘organelles’) in a cell to measure many features, which could give clues as to what’s going wrong in disease that researchers might not have considered before.
“The idea is essentially, rather than tagging your favorite protein or your favorite marker, what if we just added organelle dyes? Then we measure many features, usually about 8,000, in each cell. And as someone who was trained in molecular cell biology, when I joined the group, I was like, ‘there’s no way you learn anything from that, that’s silly.’ But it turns out that you can actually learn quite a bit from just deep measurement of organelle dyes.”
NYSCF has also approached some of its research in a ‘disease agnostic’ way, helping to identify disease features invisible to the human eye.
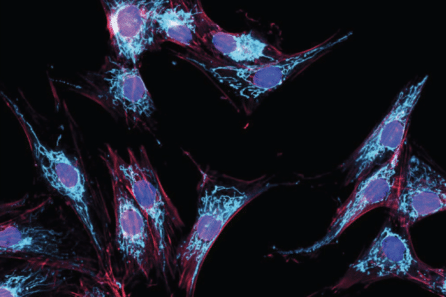
“We did this in Parkinson’s disease, where we used the robots to grow lots and lots of cells, ran Cell Painting many, many times and then imaged them and applied a deep learning model,” said Dr. Paull.
The result? Identification of new cellular hallmarks of Parkinson’s disease. The discovery of these novel disease signatures using unbiased methods, especially across patient populations, has value for diagnostics and drug discovery, even revealing new distinctions between patients.
‘Clinical Trials in a Dish’
This idea of drug screening in a dish is critical for quickly identifying the best compounds to advance to clinical trials, as well as determining which already-approved drugs could work best for each individual patient.
“What’s incredibly striking in the field of stem cell technologies is that now that we have such robust systems, we can personalize treatments to each patient in the clinic,” said Dr. Ramos. “There are folks that run a clinic and a research laboratory who have the resources and ability to see a patient, retrieve their stem cells, and potentially even test drugs to see which a patient will respond to.”
One example of this approach comes from NYSCF-funded scientists at Harvard, who discovered that an epilepsy drug could reduce hallmarks of ALS in patient brain cells made from stem cells. This work has subsequently shown promising results in clinical trials.
At NYSCF, we are currently working on a rare disease called infantile neuroaxonal dystrophy (INAD) which affects children and has similarities to Parkinson’s and Alzheimer’s. Making stem cells from INAD patients and running drug screens has helped scientists identify potential new therapies.

“These children affected by this don’t live much beyond 10 to 12 years old,” said Dr. Paull. “And the question we obviously want to answer is whether we can revert this. Can we make these sick cells appear healthy again? And so we’ve been screening an FDA-approved library of drugs on these cells, and have found compounds that do indeed show a positive effect.”
“It seemed that the knowledge we can gain from looking at stem cells was important for understanding the disease mechanisms and ultimately coming up with a more effective treatment option,” noted Leena Panwala, President of INADcure, whose daughter Ariya carries the disease. “We can take a closer look at those cells to get a better understanding of the mechanisms behind INAD at the cellular level in these children. That has never been done before for this disease.”

