Inside NYSCF’s Cell Therapy for Macular Degeneration
Age-related macular degeneration (AMD) is a leading cause of vision loss in older adults, affecting almost 20 million Americans....Age-related macular degeneration (AMD) is a leading cause of vision loss in older adults, affecting almost 20 million Americans. Harnessing the transformative power of stem cells, NYSCF scientists have been developing a cutting-edge therapy to replace the retinal cells that are lost in this disease due to aging.
NYSCF is partnering with the National Eye Institute (NEI) and surgeons at Columbia University to bring this personalized dry AMD cell therapy into the clinic in the fall of 2025. As a first step, we built an FDA-approved Good Manufacturing Practice (GMP) clean room at NYSCF where we are making the clinical-grade human cells. This state-of-the-art facility, one of only a handful in the country, coupled with NYSCF’s cell manufacturing expertise places NYSCF at the forefront of the field.
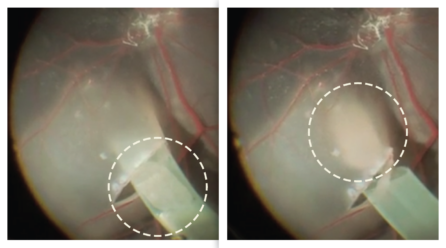
In this clinical trial, nurses at NYSCF will collect blood from each patient. NYSCF scientists will then turn their blood cells into stem cells, and then into retinal cells. These retinal cells will be placed onto a patch which will be transplanted into the eye. Because this patch is biodegradable, the surgery will leave no trace other than 100,000 new retinal cells, which will integrate into the region of the eye damaged in AMD.
The surgical team is led by Columbia University’s renowned ophthalmologist Dr. Stanley Chang, working closely with Dr. Stephen Tsang and Dr. Tongalp Tezel. This clinical trial is among the first in the world to restore vision using a patient’s own cells. This personalized approach will remove the risk of transplant rejection, precluding the need for immune suppression.
“It’s essential to have places like NYSCF which can create these clinical grade cells. It’s a capability that very few have,” said Dr. Stanley Chang