Predicting Chronic Pain in the Brain
NewsThe Context: Roughly 50 million Americans experience chronic pain (persistent or recurrent pain lasting longer than 3 months), but we do not have adequate methods for measuring or preventing it. Researchers have long suspected that chronic pain results from faulty wiring in the brain, but its relationship to brain activity versus that of the temporary pain we feel when, for example, stubbing a toe, has not been well understood.
The Study: Scientists implanted neural recording devices in the brains of four patients (three with post-stroke pain and one with phantom limb pain) to trace brain activity during chronic pain. The team identified fluctuations from a region called the orbitofrontal cortex as indicative of chronic pain. This study, led by NYSCF – Robertson Neuroscience Investigator Alumnus Edward Chang, MD, of the University of California, San Francisco, appears in Nature Neuroscience.
The Importance: This study provides the first prediction of chronic pain state based on brain activity, illuminating what the brain does during chronic pain. This could pave the way for biomarkers of chronic pain and new treatment strategies, for example, via an implant that stimulates certain brain regions to disrupt pain signaling.
“Because the way chronic pain reorganizes neural networks is so unique to each individual, we really need more personalized treatment strategies for dealing with it,” Prasad Shirvalkar, MD, PhD, associate professor of anesthesia, neurology, and neurological surgery – and first author on the study – told UCSF News. “In order to target an individual patient’s pain, we first need objective biomarkers of a subjective experience.”
How Bad Does it Hurt?
If you’ve ever gone to the doctor about an issue involving pain, you’ve probably encountered ‘the chart.’ The chart displays emojis ranging from a perfectly content smiling face to one crying out for help. But what might be one person’s ‘slightly uncomfortable-faced’ emoji might be another’s ‘pure despair’ emoji. One of the tricky facets of chronic pain is that there isn’t a good way of reporting its severity outside of subjective measures.
“There’s a big movement in the pain field to develop more objective markers of pain that can be used alongside self-reports,” Kenneth Weber, MD, a neuroscientist at Stanford University who was not involved in the study told The New York Times.
To get an objective measurement, scientists turned to the headquarters of chronic pain: the brain.
Finding Pain in the Brain
The team decided to record activity in two brain regions: the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC). The ACC is what controls the emotional component of pain, the one that makes you think ‘I hate this.’ The OFC is less studied in pain, but has connections to the ACC and is thought to possibly play a role in the cognitive side of pain, the one that makes you think ‘Oh God, this is going to hurt.’
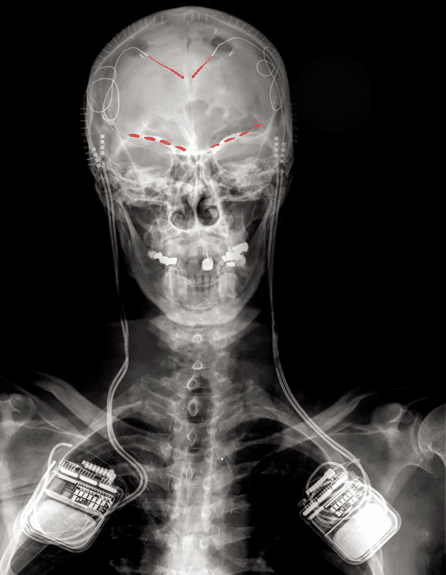
Brain recordings were taken via an implant placed in the brains of four patients – 3 with post-stroke pain and one with phantom limb pain. The patients lived their lives as they normally would, and every few hours were asked to report their pain levels. At each of these intervals, the implant would then start recording brain activity.
What the team uncovered was a unique ‘pain fingerprint’ for each patient. With machine learning models, the scientists could predict how a patient reported their pain based on activity in the brain. Surprisingly, signals from the OFC were the most predictive in this process.
“This is a big milestone because it’s the first time that neural activity related to chronic pain has been measured in the real world over a clinically relevant time period,” remarked Dr. Shirvalkar. “And while the biomarkers we found were specific to each individual, their location in the OFC appeared to be common across subjects.”
Acute vs. Chronic Pain
How similar is the brain’s experience of chronic pain to that of the acute pain we feel when we touch something hot or step on a lego? To find out, the scientists conducted a laboratory experiment with the patients in which they were exposed to a small acute pain, and their brain activity was measured.
In two of the participants, pain signals came mostly from the ACC and didn’t last as long as those found in the OFC during chronic pain, suggesting the ACC is more of the hub for acute pain.
This helps explain why many treatments for acute pain, such as opioids, do not work as well for chronic pain.
Toward Better Treatments
By understanding the ‘fingerprint’ of each person’s pain, scientists are now better equipped to create better treatments.
One idea the researchers have is to use the very same type of neural implant they employed to measure brain activity to instead deliver electrical currents to the brain that disrupt pain signals.
“The device we implanted into the participants in this study is actually a deep brain stimulation device that has the capability not only to sense and record brain activity, but also to provide electrical stimulation when needed,” explained Philip Starr, MD, PhD, professor of neurological surgery and a senior author of the study. “The hope is that by pinpointing the specific brain signals underlying someone’s pain experience, we can program the device to deliver stimulation only when those signals are detected and return the brain’s pain networks to a normal, healthy state.”
Altogether, the scientists are excited for what these findings will mean for chronic pain sufferers.
“Chronic pain manifests very differently in different people, which makes it an especially good candidate for personalized neurostimulation,” noted Dr. Chang. “This brings us a step closer to developing a new therapy for people suffering from ongoing pain.”
Journal Article:
First-in-human prediction of chronic pain state using intracranial neural biomarkers
Prasad Shirvalkar, Jordan Prosky, Gregory Chin, Parima Ahmadipour, Omid G. Sani, Maansi Desai, Ashlyn Schmitgen, Heather Dawes, Maryam M. Shanechi, Philip A. Starr & Edward F. Chang. Nature Neuroscience. 2023. DOI: https://doi.org/10.1038/s41593-023-01338-z
Top image: During the study, patients drew their own pain body diagrams. These are diagrams from four different study participants showing the location and intensity of their pain. Green equals mild pain for the participant, while red equals severe pain. From left to right: post-stroke pain, phantom limb pain, post-stroke pain, post-stroke pain. Image by Prasad Shirvalkar

