For Brain Disease Research, Patients Lead the Way
“There are 55 million people worldwide living with Alzheimer’s or other dementias, 2.8 million living with multiple sclerosis, and...
Our Approach MS News FAQs. Meet the PI For Scientists Publications
At NYSCF, we are using the power of stem cells to create the actual human cell types affected by multiple sclerosis, from people with the disease. Many current multiple sclerosis treatments target immune cells in the body to prevent them from attacking myelin – the ‘insulation’ around our neurons that helps them send signals – but other cell types within the brain are also likely involved in the disease. Our goal is to study how these cells contribute to multiple sclerosis causes and develop therapies to target them. We strive to accomplish this in several ways:
1. At the NYSCF Research Institute, we are using our own, powerful robotic system for creating stem cells. Our NYSCF Global Stem Cell Array® can rapidly, cleanly, and reproducibly create stem cells from the skin or blood of patients, and then turn them into the different brain cells implicated in multiple sclerosis: astrocytes, neurons, microglia, and oligodendrocytes.

2. Since our technology allows us to study numerous samples at a time, we are able to examine how multiple sclerosis affects different individuals and those with different subtypes of the disease. We also investigate how the genetic variation between individuals may impact their experience of MS.
3. We then study how different brain cells function, interact, and degenerate by exposing them to the type of inflammation found in multiple sclerosis and studying their response. This information can be used to develop effective therapies, including strategies to restore myelin on neurons and thus hopefully restore the function of the nervous system.
Our work largely focuses on progressive multiple sclerosis that gradually worsens even in the absence of relapses. Progressive forms of MS are more aggressive and do not respond well to current therapies.
“There are 55 million people worldwide living with Alzheimer’s or other dementias, 2.8 million living with multiple sclerosis, and...
Human stem-cell-derived astrocytes can be triggered to become toxic to neurons, opening the door for new understanding and treatment...
The Context: Astrocytes are support cells in the brain that help our neurons function properly. However, these typically helpful...
The Context: Glia – the brain’s helper cells – are often overlooked in disease research, but are known to...
Multiple sclerosis is an inflammatory and neurodegenerative disease, and it is the most common cause of non-traumatic neurological disability in young adults. In multiple sclerosis, the immune system attacks myelin, the protective coating surrounding nerve fibers that helps them send signals. This can lead to symptoms such as numbness, weakness, vision loss, tremor, fatigue, and cognitive impairment.
There are three major subtypes in which multiple sclerosis has been traditionally classified, but this definition undergoes continuous revisions to better reflect the advancements in our knowledge of the disease:
-In relapsing-remitting multiple sclerosis, a patient’s symptoms come and go, intermitted by phases of recovery.
-Secondary progressive multiple sclerosis occurs when symptoms from relapsing-remitting MS begin to persist and worsen, without a remission between the relapses.
-Primary progressive multiple sclerosis is a rare, yet severe, case in which patients show persistent accumulation of disability without necessarily undergoing the relapsing-remitting phase.
Our research focuses on the progressive forms of MS, as they are the most aggressive and do not respond as well to current therapies.
The MS Society defines a multiple sclerosis relapse as “the appearance of new symptoms, or the return of old symptoms, for a period of 24 hours or more – in the absence of an infection or a change in your core body temperature. On top of that, 30 days must have passed since your last relapse or flare up of symptoms.”
Multiple sclerosis is thought to be an autoimmune disease, as the immune system erroneously attacks myelin, the ‘insulation’ surrounding neurons that helps them send signals. Multiple sclerosis is also a neurodegenerative disease, and the pathogenic mechanisms leading to disease progression are not fully understood.
While multiple sclerosis is not directly inherited, studies suggest there is likely a genetic component to one’s risk of developing the disease. NYSCF’s research uses stem cells to tease apart these genetic risk factors so as to help develop options for early diagnosis, treatment, and/or prevention.
While there are several treatment options available for symptoms of multiple sclerosis, and highly effective therapies for relapsing/remitting MS, there is currently no cure for the progressive forms of the disease.
Multiple sclerosis was first defined and named by French neurologist Jean-Martin Charcot in 1868.

Valentina Fossati, PhD
Senior Research Investigator
The NYSCF Research Institute
Dr. Fossati leads NYSCF’s multiple sclerosis program and is a NYSCF–Druckenmiller Postdoctoral Fellow and NYSCF – Helmsley Investigator Alumna. She obtained her PhD in Biotechnology from the University of Bologna, and during her postdoctoral work at Mount Sinai School of Medicine, she studied immune system development. After being diagnosed with multiple sclerosis in 2009, Dr. Fossati shifted her research to better understanding this disease. Bringing stem cell expertise to multiple sclerosis research, she has pioneered some of the most widely used methods in the field to convert patient stem cells into all major brain cell types, with the ultimate aim of enabling drug discovery and cell replacement therapies.
Dr. Fossati’s research focuses on glial cells, including oligodendrocytes, astrocytes, microglia, to identify and target the key glia-driven pathogenic mechanisms leading to neurodegeneration and/or demyelination not only in MS, but also across neurodegenerative and neuroinflammatory diseases including Alzheimer’s disease, multiple system atrophy, and others. Dr. Fossati also pioneered studies of human models of brain cells in Low Earth Orbit, sending the first patient-derived brain organoids for long-term cultures onboard the International Space Station.
Email: vfossati@nyscf.org
Check out interviews with Valentina in NeuroCentral, MS Discovery Forum, and Cellular Reprogramming.
Our goal is to develop human induced pluripotent stem cell (iPSC) models to study pathogenic mechanisms that drive neurodegeneration in MS.

Using the NYSCF Global Stem Cell Array®, our advanced robotic technology for iPSC reprogramming, we have built the largest reported collection of iPSC lines from people with MS spanning diverse clinical subtypes as well as unaffected individuals, and we have pioneered protocols to differentiate iPSCs into glial cells, including astrocytes, oligodendrocytes, and microglia. We have also established co-culture and organoid systems to investigate the cross-talk between neurons and glia, and are developing a biomimetic platform to achieve efficient myelination in vitro and study the processes of demyelination and remyelination.
We are also investigating the roles of astrocytes and microglia in driving neuroinflammation and the degeneration of neurons, not only in MS but also in other neurodegenerative diseases such as Alzheimer’s or Multiple System Atrophy. Increasing evidence supports the existence of shared pathogenic mechanisms across these diseases, and we are thus building tools and methods that can be broadly applied within the neuroscience community.
Our group has pioneered the development of human models to study MS. We generated the first iPSC lines from people with progressive MS and, thanks to NYSCF’s automated capabilities for stem cell research, we have expanded this collection, which is now the largest biobank of iPSC lines from people with MS.
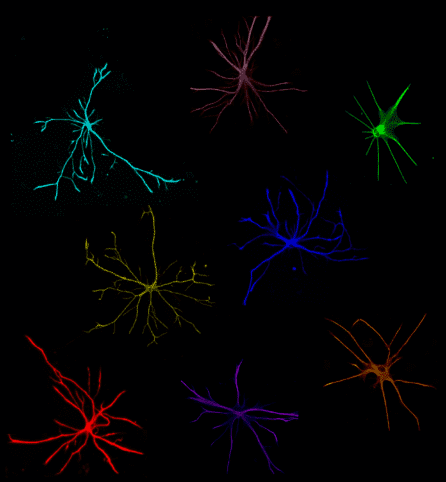
Over the past decade, we have pioneered iPSC differentiation protocols to generate the main glial cells: oligodendrocytes, astrocytes, and microglia, as well as 3D organoid cultures that integrate all these cell types with neurons. These include some of the most efficient and widely used protocols that are being applied to study neurodegenerative diseases. We have applied these protocols to model disease-relevant crosstalk between cell types. One example is the triggering of reactive substates of astrocytes in response to inflammatory signals that lead to alterations in astrocyte function, ranging from neurotoxicity to neuroprotection.
In our recent preprint, we performed single-cell transcriptomic analysis of brain cells derived from MS patient iPSC models, revealing glia-intrinsic differences in MS cultures. This opens up new possibilities for the understanding and treatment of MS.
Our goal in developing methods for iPSC modeling is to make them as efficient and reproducible as possible so that the field can benefit. We have published our differentiation protocols for glia, co-cultures, and organoids,, providing all details to enable reproducibility, and have worked with many labs worldwide to help them establish these methods:
Datasets:
We recently made a collection of iPSC lines from people with MS available through the NYSCF repository.
We are committed to collaborating with the community to ensure that our iPSC models are useful and to expand their ability to capture critical disease phenotypes and advance new therapies. Please reach out if you are interested in working with us. The following are some of our ongoing collaborations:
Proteomic profiling of interferon-responsive reactive astrocytes in rodent and human
Priya Prakash, Hediye Erdjument-Bromage, Michael R. O’Dea, Christy N. Munson, David Labib, Valentina Fossati, Thomas A. Neubert, Shane A. Liddelow. Glia. 2023. DOI: https://doi.org/10.1002/glia.24494
Longitudinal scRNA-seq analysis in mouse and human informs optimization of rapid mouse astrocyte differentiation protocols
Paul W Frazel, David Labib, Theodore Fisher, Ran Brosh, Nicolette Pirianian, Anne Marchildon, Jef D Boeke, Valentina Fossati, Shane A Liddelow. Nature Neuroscience. 2023. DOI: https://doi.org/10.1038/s41593-023-01424-2
Patient iPSC models reveal glia-intrinsic phenotypes in multiple sclerosis
Clayton BLL, Barbar L, Sapar M, Rusielewicz T, Kalpana K, Migliori B; NYSCF Global Stem Cell Array® Team; Paull D, Brenner K, Moroziewicz D, Sand IK, Casaccia P, Tesar PJ, Fossati V. bioRxiv. 2023 Aug 2:2023.08.01.551553. doi: 10.1101/2023.08.01.551553. Preprint. PMID: 37577713
Reactive Astrocytes Derived From Human Induced Pluripotent Stem Cells Suppress Oligodendrocyte Precursor Cell Differentiation
Matthew D Smith, Xitiz Chamling, Alexander J Gill, Hector Martinez, Weifeng Li, Kathryn C Fitzgerald, Elias S Sotirchos, Dorota Moroziewicz, Lauren Bauer, Daniel Paull, Marjan Gharagozloo, Pavan Bhargava, Donald J Zack, Valentina Fossati, Peter A Calabresi. Front Mol Neurosci (May, 2022). DOI: doi.org/10.3389/fnmol.2022.874299
Proteomic Alterations and Novel Markers of Neurotoxic Reactive Astrocytes in Human Induced Pluripotent Stem Cell Models
David Labib, Zhen Wang, Priya Prakash, Matthew Zimmer, Matthew D Smith, Paul W Frazel, Lilianne Barbar, Maria L Sapar, Peter A Calabresi, Junmin Peng, Shane A Liddelow, Valentina Fossati. Front Mol Neurosci (May, 2022). DOI: https://doi.org/10.3389/fnmol.2022.870085
Human Pluripotent Stem Cell Differentiation to Microglia
Laraib Ijaz, Madhura Nijsure, Valentina Fossati. Methods in Molecular Biology. (March, 2021). DOI: doi.org/10.1007/7651_2021_359
Isolation of Human CD49f+ Astrocytes and In Vitro iPSC-Based Neurotoxicity Assays
Lilianne Barbar, Tomasz Rusielewicz, Matthew Zimmer, Kriti Kalpana, Valentina Fossati. STAR Protocols. 2020. DOI: 10.1016/j.xpro.2020.100172
CD49f is a novel marker of functional and reactive human iPSC-derived astrocytes
Lilianne Barbar, Tanya Jain, Matthew Zimmer, Ilya Kruglikov, Jessica Sadick, Minghui Wang, Kriti Kalpana, Indigo V.L. Rose, Suzanne R. Burstein, Tomasz Rusielewicz, Madhura Nijsure, Kevin A Guttenplan, Angelique di Domenico, Gist Croft, Bin Zhang, Hiroko Nobuta, Jean M. Hébert, Shane A. Liddelow, Valentina Fossati. Neuron. 2020. DOI: https://doi.org/10.1016/j.neuron.2020.05.014
Mechanosensitivity of Human Oligodendrocytes.
Espinosa-Hoyos D, Burstein SR, Cha J, Jain T, Nijsure M, Jagielska A, Fossati V, Van Vliet KJ. Front Cell Neurosci. 2020 Jul 24;14:222. doi: 10.3389/fncel.2020.00222. eCollection 2020. PMID: 32848617
A metabolic perspective on CSF-mediated neurodegeneration in multiple sclerosis
Maureen Wentling, Carlos Lopez-Gomez, Hye-Jin Park, Mario Amatruda, Achilles Ntranos, James Aramini, Maria Petracca, Tom Rusielewicz, Emily Chen, Vladimir Tolstikov, Michael Kiebish, Valentina Fossati, Matilde Inglese Catarina, M Quinzii, Ilana Katz Sand, Patrizia Casaccia. Brain. 2019. doi: https://doi.org/10.1093/brain/awz201
Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis
Nicaise AM, Wagstaff LJ, Willis CM, Paisie C, Chandok H, Robson P, Fossati V, Williams A, Crocker SJ. Proc Natl Acad Sci U S A. 2019 Apr 30;116(18):9030-9039. doi: 10.1073/pnas.1818348116. Epub 2019 Mar 25. PMID: 30910981
Cerebrospinal fluid biomarkers link toxic astrogliosis and microglial activation to multiple sclerosis severity
Ruturaj Masvekar, Tianxia Wu, Peter Kosa, Christopher Barbour, Valentina Fossati. Multiple Sclerosis and Related Disorders. 2018. DOI: https://doi.org/10.1016/j.msard.2018.11.032
Induction of myelinating oligodendrocytes in human cortical spheroids.
Madhavan M, Nevin ZS, Shick HE, Garrison E, Clarkson-Paredes C, Karl M, Clayton BLL, Factor DC, Allan KC, Barbar L, Jain T, Douvaras P, Fossati V, Miller RH, Tesar PJ. Nature Methods. doi: 10.1038/s41592-018-0081-4
Directed differentiation of human pluripotent stem cells to microglia.
Douvaras P, Sun B, Wang M, Kruglikov I, Lallos G, Zimmer M, Terrenoire C, Zhang B, Gandy S, Schadt E, Freytes DO, Noggle S, Fossati V. Stem Cell Reports. 2017. doi: 10.1016/j.stemcr.2017.04.023
Epigenetic Modulation of Human Induced Pluripotent Stem Cell Differentiation to Oligodendrocytes
Douvaras P, Rusielewicz T, Kim KH, Haines JD, Casaccia P, Fossati V. International Journal of Molecular Sciences. 2016. DOI: 10.3390/ijms17040614.
Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells
Douvaras P, Fossati V. Nature Protocols. 2015. DOI: 10.1038/nprot.2015.075.
Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells
Douvaras P, Wang J, Zimmer M, Hanchuk S, O’Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Stem Cell Reports. 2014 Aug 12;3(2):250-9. doi: 10.1016/j.stemcr.2014.06.012. Epub 2014 Jul 24. PMID: 25254339