Consortium Including NYSCF Scientists Receives Grant Award to Study Parkinson’s Disease Risk Factors
NewsA multi-institutional team of scientists led by Lorenz Studer, MD (Memorial Sloan Kettering Cancer Center) and including Gist Croft, PhD (The NYSCF Research Institute), NYSCF – Robertson Stem Cell Investigator Vikram Khurana, MD, PhD (Brigham and Women’s Hospital), Jian Peng, PhD (University of Illinois at Urbana-Champaign), and Joseph Powell, PhD (Garvan Institute of Medical Research) have received a grant from the Aligning Science Across Parkinson’s (ASAP) initiative to use stem cells to study how risk factors accumulate and interact to drive Parkinson’s disease (PD). The team will receive 9 million over 3 years to take a comprehensive look at the interplay between genetics, aging, and different brain cell types underlying individual risk for PD in a project entitled, “Defining the cellular and molecular determinants of variable genetic penetrance in Parkinson’s disease.”
“NYSCF is honored to be part of this innovative, highly collaborative effort to use the power of stem cells to answer critical questions about the mechanisms of Parkinson’s disease,” remarked NYSCF CEO, Susan L. Solomon, JD. “We are especially pleased to be working with our long-time collaborator, Dr. Lorenz Studer, and NYSCF – Robertson Stem Cell Investigator Dr. Vikram Khurana on this endeavor.”
The international team integrates diverse expertise that will be key for tackling this complex disease:
- Dr. Studer will study how age-related risk factors combine with PD genetic risk factors to trigger disease
- Dr. Croft will examine how interactions between the brain’s various cell types and neuroinflammation can factor into PD
- Dr. Khurana will lead functional genomic studies that dissect mechanisms through which different combinations of genetic risk factors lead to PD
- Dr. Powell will lead high-throughput cellular genomic experiments, which allow scientists to study individual genetic manipulation and expression data of stem cells pooled in the thousands
- Dr. Peng will apply machine learning to all of this data to identify patient subgroups and reveal new disease mechanisms
By receiving this grant, the team joins the ASAP Collaborative Research Network, an effort to support international, multidisciplinary, multi-institutional research teams to address key knowledge gaps in the basic disease mechanisms that contribute to PD development and progression. ASAP is working with implementation partner The Michael J. Fox Foundation for Parkinson’s Research to issue the grants.
The Mystery of What Drives PD
Parkinson’s disease (PD) is an especially tricky disease to study because while scientists have identified many genetic risk factors, many people who carry these risk factors do not develop PD — a phenomenon known as ‘variable penetrance.’ It is still unclear how exactly PD is triggered and how brain cells become diseased as patients age. These gaps in knowledge are a major reason why there are no disease-modifying treatments that can slow, stop, or reverse PD. To develop effective treatments, we need to better understand the numerous complex factors that lead to the disease.
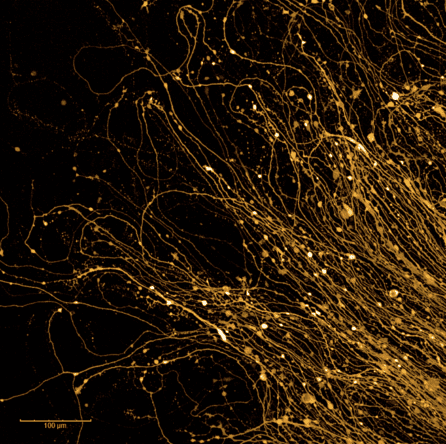
“We believe that single genetic risk factors are probably not enough to cause PD on their own,” said Dr. Studer, a founding member of NYSCF’s medical advisory board and Director of the Center for Stem Cell Biology at Memorial Sloan Kettering Cancer Center. “More likely, PD is triggered by a combination of genetics, age-related factors, and their effects in different brain cells. Stem cells give us the perfect opportunity to look at the interplay of these factors in the different cell types of the brain.”
“We think a number of genetic factors tied to the fundamental protein-aggregation pathology that occurs in PD work in combination to increase disease risk,” agreed Dr. Khurana, Chief of the Division of Movement Disorders at Brigham and Women’s Hospital and an Assistant Professor of Neurology at Harvard Medical School. “This project will allow us to investigate how these genetic variations actually impact cells in the brain, bridging causation to an understanding of actual disease mechanism.”
Peeking Inside a Parkinson’s Brain
Taking advantage of genetically diverse patient stem cell lines already generated by these labs, the scientists will turn them into the different brain cells implicated in PD: neurons, microglia, and astrocytes. This will allow the scientists to study exactly how the actual human cells impacted by PD behave, showing how genetic risk factors, the aging process, and these different cell types interact to trigger disease.
“While we have known for decades that specific neurons die in PD, we have only recently learned that their interactions with other brain cells, astrocytes and microglia, are critical,” explained Dr. Croft, Senior Research Investigator in Parkinson’s Disease and Neurodegeneration at the NYSCF Research Institute. “Patient stem cell models enable us to mix and match these cell types, to reveal their independent and interactive roles in PD with unprecedented resolution.”
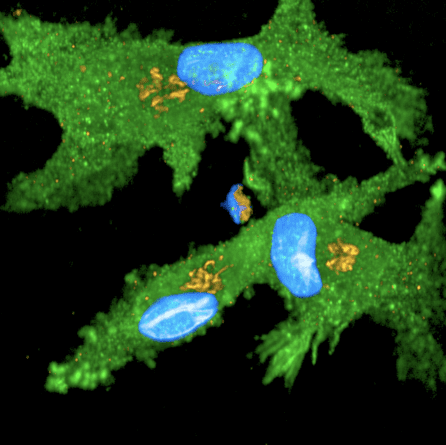
“The technologies we have now for investigating biology at the single-cell level are incredibly sophisticated,” added Dr. Powell, Head of the Garvan-Weizmann Centre for Cellular Genomics at the Garvan Institute of Medical Research. “This will allow us a comprehensive look at the behavior of individual patient brain cells to help understand exactly where things go wrong in PD.”
With a better understanding of which risk factors contribute to disease progression in which subgroups of patients, the researchers will then build a computational network model of the factors that cause PD.
“This artificial-intelligence-driven model will help us identify new targets for PD drugs or gene therapies,” said Dr. Peng, an Assistant Professor of Computer Science at University of Illinois. “Once we have those, we can evaluate therapeutic options in our patient-derived stem cells, giving us a better idea of what types of treatments will work for which subgroups of patients.”
Paving the Way for Precision Medicine
The team hopes that their study will provide an entirely new level of understanding of how the interplay between genetics, different brain cells, and aging shapes individual disease risk.
“With more information about the complex mechanisms that make someone at a higher risk for developing PD, we can start working toward strategies for early diagnosis and personalized therapeutic interventions that halt or reverse the disease,” said Dr. Studer. “I am delighted to be working with such an exceptional, collaborative team to take on these important challenges and ultimately improve the lives of patients.”

