What are Stem Cells, and How Can They Accelerate Better Treatments for Disease?
Alzheimer’s, Parkinson’s, multiple sclerosis, cancer: they’re all diseases that are, to this day, largely a mystery, and treatment options...Alzheimer’s, Parkinson’s, multiple sclerosis, cancer: they’re all diseases that are, to this day, largely a mystery, and treatment options are scarce. But thanks to stem cells, these diseases, along with countless others, may finally be cracked. Better treatments, and even cures, could be reaching patients sooner than you think thanks to these revolutionary little cells.
NYSCF’s Senior Vice President of Research Scott Noggle, PhD, recently gave a crash course on the basics of stem cells and why they are a breakthrough tool for medical research.
What are stem cells?
“Stem cells are the superheroes of the body,” explained Dr. Noggle. “They’re unique cells that can become any cell type – brain cells, heart cells, lung cells, and everything else. They can also self-renew. We could fill up this entire room with stem cells if we gave them the right signals to form more of themselves.”
NYSCF’s research employs a special type of stem cell called ‘induced pluripotent stem cells.’
“At NYSCF, we make induced pluripotent stem cells, which are stem cells made from skin or blood that carry the genetics of the person from whom they were made,” said Dr. Noggle. “And this is really important for disease research, because it lets us essentially create an avatar of someone in a dish.”
Thanks to NYSCF’s state-of-the-art robotic platform, the process of making stem cells is faster and more efficient than ever.
“We have developed a set of robotic systems that we call the NYSCF Global Stem Cell Array®, and they basically automate the whole process of making stem cells from skin or blood and turning them into other cell types,” noted Dr. Noggle. “With the Array, we can make many hundreds to thousands of stem cell lines at a time, allowing us to scale up our studies and look at populations of people rather than just cells from one or two.”
How can stem cells be used to teach us about human diseases?
“Fundamentally we cannot treat what we don’t understand. We need to be able to look at the actual human cells affected by different diseases to investigate what is going wrong,” remarked Dr. Noggle.
“Many of these cells are not cells that we would typically have easy access to. For example, I can’t exactly ask for some of your brain cells so I can study brain diseases, but with stem cells, we can make them.”
NYSCF scientists do this, for example, with Alzheimer’s disease, and can use these cells to tease apart the roots of the disease.
Brains of Alzheimer’s patients often present with plaques and tangles – buildups of proteins that accumulate in the brain. However, this likely isn’t the whole story.
“Sometimes we find plaques and tangles in patients that never developed clinical Alzheimer’s disease,” said Dr. Noggle. “This disease is turning out to be very complex with many genetic factors and different subtypes. So, we have been employing stem cells as a tool to try to understand how our different genetics can result in degeneration and influence different aspects of Alzheimer’s disease.”
It’s also important to look at all the different cell types of the brain, and with stem cells, scientists can make all the major types: neurons (the communicators), astrocytes (the surveyors), oligodendrocytes (the insulators), and microglia (the immune cells of the brain).
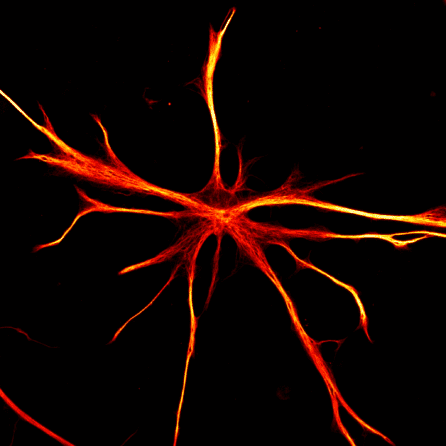
“All of these cells work together in the brain, and we think that the way that they work together is critical for how Alzheimer’s develops,” shared Dr. Noggle. “We can even create brain organoids, which are basically little aggregates of tissue made from all the different cell types. We use these to study how these cells communicate with each other and what might go wrong to lead to the development of Alzheimer’s.”
“Trying to study Alzheimer’s disease in a dish is challenging, however, because Alzheimer’s is a disease of aging,” he continued. “We wanted to know if the cells that we’re making in the laboratory actually reflect the biology of Alzheimer’s as it exists in the brains of people with the disease.”
In collaboration with scientists at Rush University Medical College and Harvard Medical School, Dr. Noggle’s team created neurons from the stem cells of 53 deceased individuals representing the spectrum of brain aging from healthy to Alzheimer’s disease. The researchers found that these stem-cell-derived neurons showed the same hallmarks as the actual brain tissue from these individuals.
“Remarkably, the stem-cell-derived brain cells that we studied from these individuals had characteristics that match up to what we actually see in those people’s brains,” remarked Dr. Noggle. “So we have a lot of confidence that the disease process that we’re looking at in the laboratory is actually reflected in real disease biology in the brain, which is very exciting.”
How can stem cells accelerate and improve drug discovery?
“A lot of the drugs are developed using different types of animal models, and in many cases, these animals have not really been predictive of the way that these diseases and drugs work in humans,” noted Dr. Noggle.
Because of this, many drugs don’t make it through clinical trials.
“80 to 90% of drugs fail clinical trials. And for the ones that do make it, the process is long and costly, and many of the drugs don’t actually work well in all patients.”
With stem cells, scientists can test drugs on the actual human cells affected by a disease.
“Labs such as that of Kevin Eggan, PhD, and NYSCF – Robertson Stem Cell Investigator Brian Wainger, MD, PhD, have been using motor neurons derived from the stem cells of ALS patients to see if they could understand what might be going wrong in the brain,” said Dr. Noggle.
“They noticed that the neurons they made from ALS patient stem cells were hyperactive. They then did a drug screen to look at whether any existing drugs could reverse this, and they found that an epilepsy drug called ezogabine could do this. They’ve now performed clinical trials to show that in patients, this drug actually could quell the overactive motor neurons.”
Dr. Noggle also wants to make sure the drugs scientists find are safe. One of the major reasons why drugs fail is that they’re toxic to different cells in the body.
“We can make things like cardiomyocytes [heart cells] from the stem cells. One of the main complications with many drugs is that they affect the heartbeat. When we make heart cells from the stem cells, they will actually beat in the dish and reflect the biology of that person, so we can know ahead of time whether or not they might have a toxic reaction to one of the drugs that we develop.”
How can stem cells be used to create therapies?
The other way that scientists are interested in using stem cells is to replace dead or dying cells that might occur in different types of disease. NYSCF is currently doing this by creating a cell therapy to treat age-related macular degeneration (AMD), the world’s leading cause of blindness.
“There’s a type of cell in the back of the eye called the retinal pigmented epithelial cells, or ‘RPE cells’ that die in patients with AMD,” explained Dr. Noggle. “What we’re trying to do is see if we can replace those dead and dying RPE cells with healthy ones made from stem cells. We can create sheets containing healthy RPEs to be surgically implanted into the eye to restore vision.”
This work is happening in collaboration with surgeons at Columbia University – NY Presbyterian Hospital led by Stanley Chang, MD, Stephen Tsang, MD, and Tongalp Tezel, MD. The clinical-grade cells are being produced in NYSCF’s state-of-the-art Good Manufacturing Practices (GMP) facility, which was completed last summer.
Altogether, Dr. Noggle is optimistic about the future of stem cells in creating new treatments.
“I am really excited about the ongoing therapeutic work that’s actually starting to come to fruition – things like the ALS drug I mentioned,” Dr. Noggle noted. “I think we’re going to start seeing more of this by using stem cells as a tool. I’m really excited about the prospects of that over the next few years.”

