Experts Discuss the Latest in COVID-19 Research: Everything from Immunity to Stem Cells to Vaccines
News VideoIt has been six months since the COVID-19 pandemic hit the United States at full force, leaving millions sick, out of work, and desperate for new solutions. While infection rates have thankfully lowered in New York lately, the virus continues to spread across the country, and the urgent need for research, treatments, and vaccines remains.
The NYSCF Innovator community is hard at work on this issue, and our scientists at the NYSCF Research Institute are creating stem cell models of COVID-19 to accelerate research worldwide. In a recent panel discussion, NYSCF community experts gathered to discuss the current state of COVID-19 research and what’s on the horizon.
The discussion was moderated by NYSCF’s Associate Vice President of Scientific Outreach Raeka Aiyar, PhD, and featured NYSCF Innovators Deepta Bhattacharya, PhD, Shuibing Chen, PhD, Larry Luchsinger, PhD, and Scott Noggle, PhD.
Are recovered patients immune to COVID-19?
An open, and concerning, question has been whether recovered COVID-19 patients retain any kind of long-term immunity to the infection, given anecdotal reports of reinfection. The encouraging news is that, yes, the immune system does appear to work as it should in most people – creating antibodies that remain present in the blood at low levels for months after patients recover from COVID-19, according to research by Dr. Bhattacharya, a NYSCF – Robertson Stem Cell Investigator Alumnus and Associate Professor of Immunobiology at the University of Arizona
“How long [immunity] will last is hard to tell. There are no shortcuts for that,” explained Dr. Bhattacharya. “We basically just have to track the data in the coming months and years and see how it goes. But if it’s anything like the first SARS coronavirus, I think we would expect it to last for at least a few years.”
“We can quantify antibody responses in individual patients through [blood] plasma, but we just don’t know what that absolute threshold is,” noted Dr. Luchsinger, a NYSCF – Druckenmiller Fellow Alumnus and Principal Investigator at the New York Blood Center. “We defer to the law of large numbers: more is probably good. Hopefully, the therapeutics and vaccines that are coming out will make it less important to know about antibody levels, because they will protect you no matter what.”
In addition to antibodies, immune cells called T cells can play a role in fighting off foreign invaders, but measuring their impact on immunity isn’t as straightforward.
“The T cells in particular are somewhat complicated because whether they protect or how much they protect is unclear. You can have some cross-reactive T cells from prior coronavirus infections, but it certainly doesn’t seem to be enough to prevent you from getting infected,” said Dr. Bhattacharya. “A lot of those cells are resident in the lung and not as easily measurable as in the blood. So, there’s multiple layers…[in addition to antibodies] there are backup layers of these memory cells that can conceivably help attenuate disease.”
What is convalescent plasma, and how does it factor into research and treatment?
A lot of what we understand about immunity to COVID-19 comes from studies of convalescent plasma – the yellow, liquid part of the blood that remains when the cells are removed – which Drs. Bhattacharya and Luchsinger are both conducting. Convalescent plasma contains antibodies and several other indicators of the body’s response to COVID-19, and likely holds clues as to why certain patients have severe reactions to infection.
“My lab is interested in whether there are molecules in the plasma that could inform us as to whether you’d be at risk for having an adverse reaction, or would be able to make a stronger antibody response than somebody else,” said Dr. Luchsinger. “These molecules could then be measured with a simple blood draw to predict individual risk.”
Convalescent plasma has been used historically in attempts to treat many different infectious diseases, with the goal of introducing antibodies from a recovered patient into a new patient to help them fight off a pathogen. Recently, convalescent plasma received a controversial emergency use authorization (EUA) from the FDA as a treatment for COVID-19. The panelists discussed several caveats of this approach.
“Most of the evidence that’s out there overwhelmingly supports the idea that plasma transfusions are relatively safe. The real question is the efficacy,” emphasized Dr. Luchsinger. “Is there a guarantee or a reasonable expectation that your transfusion will work for you? And that’s where the data is unclear.”
Dr. Bhattacharya pointed out that administering convalescent plasma to patients already experiencing severe symptoms is unlikely to be effective.
“We’ve learned that you really need to get [convalescent plasma] administered early,” he said. “If you give it late when someone’s already in the ICU, then it’s more the immune system that’s causing the problems than the virus per se.”
Another issue is that individual differences in the contents of convalescent plasma samples make it difficult to predict their effects on different patients.
“The thing that people have to understand is that each donor is going to be different, right? The antibody responses are going to be different. The cytokine profile of those plasma samples is going to be different,” noted Dr. Luchsinger. “And that is, I think, the most important thing to communicate. The differences within the samples themselves will be the variance that we’re seeing in the therapy.”
Dr. Bhattacharya is also concerned that the EUA for convalescent plasma makes it difficult to do clinical trials to fully assess its efficacy as well as trials for monoclonal antibodies – single types of antibodies that target particular parts of the virus to prevent it from entering cells.
“I had always envisioned and hoped that the monoclonal antibody therapies would start to replace the convalescent plasma, because it’s so much easier to dose. You know exactly what you’re putting in,” he said. “But a lot of those trials are having trouble enrolling people, and if everyone is getting convalescent plasma, it’s going to kill that in its tracks. I think that we need to take a step back and look at the broader landscape and think about what convalescent plasma is doing or not doing, and whether it’s getting in the way of something that’s more likely to work.”
How are stem cells helping us understand COVID-19 and find new treatments?
A major challenge in treating COVID-19 is that it can affect and damage many different tissues in the body. Stem cells allow scientists to create the different cell types impacted by COVID-19, infect them with the virus, and study their reactions – an approach that Drs. Noggle and Chen are collaborating on.
“Scott and I work together to create these stem cell models and use them to study which organs might be permissive [to SARS-CoV-2 infection]. One surprising result from our stem cell models is that pancreatic beta cells [the cells that die or malfunction in diabetes] are very permissive to SARS-CoV-2 infection,” noted Dr. Chen, an Associate Professor of Surgery and of Biochemistry at Weill Cornell Medical College. “We’re also seeing that COVID-19 may be a trigger or direct cause of diabetes. There is a case report from Europe where one teenager was infected and several weeks later was diagnosed with type 1 diabetes.”
At the NYSCF Research Institute, scientists are also creating organoids, 3D clusters of human tissue made from stem cells, to study how the SARS-CoV-2 virus infects cells and test drugs.
“Over the past few months, [we have] been creating human lung organoid models from stem cells as a resource for the community, because most research into this coronavirus is done using non-human cells that are not lung cells,” explained NYSCF CEO Susan L. Solomon.
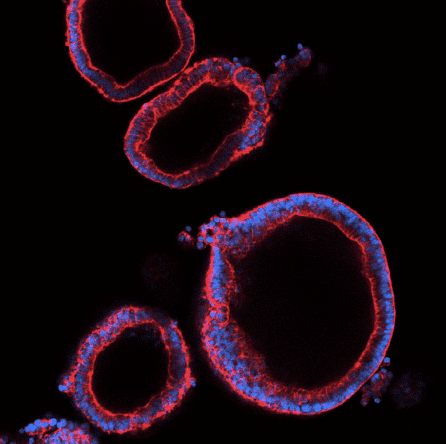
“Stem cells give us a tool to interrogate what happens over and over again in different tissues because we can create all cell types in the body, and in particular, almost all of the different cells that we think have the capability of being infected by SARS-CoV-2,” noted Dr. Noggle. “We can understand what drugs might be able to block different points of entry of the virus into the cell, or the biology of the replication of that virus inside of the cell.”
Stem cell models of COVID-19 can also serve as testing material for drugs with the potential to stop or prevent infection.
“We find that the lung organoid can mimic the body’s response to infection. After screening around 1,200 compounds on these organoids we have some very promising drug candidates, one of which turned out to be a cancer drug,” added Dr. Chen.
Stem cells can also be used to understand individual responses to COVID-19 and why some patients experience more severe reactions than others.
“In the vast majority of cases, people have a very normal immune response, but there’s still a large number of people that then go on to have what seems to be an abnormal immune response or different responses in different tissues that cause severe disease and then eventually, and in many cases, death,” said Dr. Noggle. “What we don’t have are good ways of predicting who is going to be susceptible to those more severe outcomes. So, one of our interests is to use stem cells as a tool to understand how our different genetics might impact some of that process.”
In collaboration with NYSCF, Dr. Luchsinger is aiming to use convalescent plasma and stem cells to study disease severity.
“We want to use our convalescent plasma donors as a resource for developing a COVID-19 disease model platform. So, the New York Blood Center has retrospectively sent out surveys to all of our donors and asked for symptomatology and medical data regarding their experience with COVID-19,” explained Dr. Luchsinger. “We will then choose patients that had a particular kind of reaction and send their frozen blood cells to NYSCF to create a disease model. We will then use these to see if there’s any tissue or any gene expression signature that may help explain why they responded to the virus the way they did.”
How close are we to a vaccine, and what does the road ahead look like?
The good news is that several vaccines are currently in the final phase (phase 3) of clinical trials, and the results of these vital tests should be clear in the near future.
“I am very optimistic that from an efficacy standpoint, [the vaccines] will certainly reduce disease severity. I think some of them will likely provide sterilizing immunity. Now the key is going to be looking at adverse events as we go forward,” noted Dr. Bhattacharya. “We’re going to start getting some data back in the next few months, and I don’t think it’s out of the realm of possibility that something will achieve approval by the end of the year.”

All of the panelists share hope that exciting new developments are on the way.
“I’m really happy that we have this repository of stem cell lines at NYSCF that carry a lot of the genetic variants that have been associated with severe COVID-19,” remarked Dr. Aiyar. “We’re able to start making models out of them right away to start understanding why this virus is deadly to certain individuals, which is such an important question.”
“Last time we had this panel discussion [in April], New York City was still locked down, and we still had hundreds of patients in the hospital and ICU. Right now, we are much better off,” added Dr. Chen. “We have one drug already approved, and we know more about antibodies and vaccines.”
For Dr. Noggle, approaching the virus from many different angles through widespread collaboration gives him hope.
“I think the way forward is multiple shots on goal,” he said. “And I think what’s encouraging is the speed with which the scientific community came together very early on to address this pandemic, each from our own different areas of expertise in a unified effort.”
Watch the full discussion below.

